 |
|
Contact Information
Office: MEYR 549D
Phone: 410-455-2264
Email: mptaszek@umbc.edu
Ptaszek CV
|
Associate Professor
Professional Interests
Our research interests are centered on the development of novel molecular arrays capable of absorbing and converting light in the deep-red and near-IR spectral windows. We are particularly focusing on the chemistry and photochemistry of hydroporphyrins: that are tetrapyrrolic macrocycles, which are synthetic analogues of photosynthetic pigments: chlorophylls and bacteriochlorophylls. We believe, that the very rich chemistry and photochemistry of hydroporphyrins, which are still not broadly explored, give an opportunity to create molecules with unprecedented optical and photochemical properties, which will make a significant impact of diverse areas of their application. The primary areas of intended application for such molecules are medicinal diagnosis, specifically fluorescence sensing and imaging, as well as phototherapy (specifically photodynamic therapy). However, the fundamental research performed in my lab has much broader impact on other areas, including artificial photosynthesis, light-harvesting arrays, solar energy conversion, and photocatalysis.
I. New fluorophores for in vivo bioimaging. Fluorophores for in vivo applications need to have absorption and emission wavelength between 700-900 nm, high brightness, large Stokes shift (~50 nm), high chemical and photochemical stability, low cytotoxicity, water solubility, cell permeability, etc. In addition, it is highly desirable to have fluorophores with narrow (10-20 nm) emission band, which is beneficial for simultaneous, multicolor detection of various factors. Our group focuses on the design, synthesis and characterization of tetrapyrrolic macrocycles (chlorins and bacteriochlorins) for application in fluorescent bioimaging. Chlorins and bacteriochlorins exhibit intense, narrow emission band in the optimal spectral window for in vivo application (650-850 nm). Our efforts focus on the preparation of water soluble, target-specific, tetrapyrrolic derivatives with improved photochemical properties (high qantum yield of fluorescence, large Stokes shift, and low photocytotoxicity).
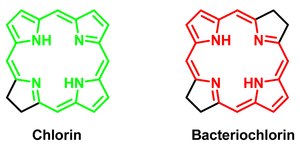 |
| Generic structures of chlorin and bacteriochlorin |
II. Strongly conjugated hydroporphyrin arrays – novel near-IR absorbing chromophores with diverse photonic applications. The goal of this project is to is to develop an understanding of structure-photophysics relationships in conjugated bacteriochlorin arrays, and to establish their feasibility for ultimately developing activatable long-wavelength fluorophores and singlet oxygen photosensitizers with potential in vivo imaging and therapeutic utility. We proposed to examine strongly conjugated bacteriochlorin dyads, i.e. dyads, where two identical bacteriochlorin subunits are connected with the linker that assures a strong electronic communications between macrocycles. In such arrays the electronic conjugation is expanded onto two or more porphyrinic subunits. As a consequence, the optical properties of such arrays are not the simple sum of the properties of their porphyrinic components, but are extensively altered. While, there are a broad data for analogous porphyrin dyads, to the best of our knowledge no prior research on the strongly conjugated bacteriochlorin dyads were reported. Our initial hypothesis was that the arrangement of BChls into strongly conjugated arrays would provide the way for extensive modulation of their optical and photochemical properties. In particular strongly conjugated BChl arrays will exhibit (a) bathochromic shift of the long-wavelength absorption and emission bands above 800 nm; (2) broad tunability of absorption and emission wavelengths, by changing the electronic properties of the conjugate linking moiety; (c) increased quantum yield of fluorescence; and (d) decreased quantum yield of ISC. The strongly conjugated bacteriochlorin arrays are now being examined as latent fluorophores and singlet oxygen photosensitizers, activatable by well-defined chemical stimulai.
III. Hydroporphyrin energy transfer arrays for multicolor fluorescence guided surgery. The aim of this project is to develop a novel generation of fluorescent probes to improve tumor visualization during surgery. Our collaborative research withy Dr. Hisataka Kobayashi group (NCI NIH) has demonstrated that bacteriochlorin-galactosylated human serum albumin conjugates visualize in vivo peritoneal ovarian cancer metastases with both great selectivity and great sensitivity. The high selectivity and sensitivity of this probes result from quenching of the bacteriochlorin fluorescence upon attachment to a protein, and fluorescence activation occurring only in the target cells. Moreover, we have shown that bacteriochlorin enables differentiation between tumors located on the surface and in deep tissue because of its ability to be excited by both green and near-IR light. In addition, bacteriochlorins, due to their exceptionally narrow emission bands, with wavelength tunability across the near-IR region (700-800 nm) can be used for multicolor simultaneous detection of multiple targets. We are preparing and optimizing a family of hydroporphyrin energy-transfer triads; where two common energy donors enables excitation with common green and deep-red wavelengths, and bacteriochlorins, equipped with the different sets of auxochromes will emit well-resolved near-IR bands.
Selected Publications
(1) Meares, A.; Yu, Z.; Bhagavathy, G. V.; Satraitis, A.; Ptaszek, M. Photoisomerization of Enediynyl Linker Leads to Slipped Co-facial Hydroporphyrin Dimers with Strong Through-Bond and Through-Space Electronic Interactions. J. Org. Chem. 2019, 84, 7851- 7862.
(2) Ogata, F.; Nagaya, T.; Maruoka, Y.; Akhigbe, J.; Meares, A.; Lucero, M.; Satraitis, A.; Fujimura, D.; Okada, R.; Inagaki, F.; Choyke, P.; Ptaszek, M.; Kobayashi, H. Activatable Near-Infrared Fluorescence Imaging Using PEGylated Bacteriochlorin-Based Chlorin and BODIPY-Dyads as Probes for Detecting Cancer. Bioconjugate Chem. 2019, 30, 169-183.
(3) Tivari, V.; Matutes, Y. A.; Konar, A.; Yu, Z.; Ptaszek, M.; Bocian, D. F.; Holten, D.; Kirmaier, C.; Ogilvie, J. P. Strongly Coupled Bacteriochlorin Dyad Studied Using Phase-Modulated Fluorescence-Detected Two-Dimensional Electronic Spectroscopy. Opt. Express, 2018, 26, 22327-22341.
(4) Meares, A.; Bhagavathy, G. V.; Zik, S. R.; Gallagher, T. Expanding π−Conjugation in Chlorins Using Ethenyl Linker. J. Org. Chem. 2018, 83, 9076-9087.
(5) McLeese, C.;. Yu, Z.; Esemoto. N. N.; Kolodziej, C.; Maiti, B.; Bhandari, Dunietz, B. D.; Burda, C.; Ptaszek, M. Excitonis Interactions in Bacteriochlorin Homo-Dyads Enable Ultrafast Charge Transfer: A Novel Approach to the Artificial Photosynthetic Special Pair. J. Phys. Chem. B, 2018, 122, 4131-4140.
(6) Esemoton, N. N.; Satraitis, A.; Wiratan, L.; Ptaszek, M. Symmetrical and Nonsymmetrical Meso–Meso Directly Linked Hydroporphyrin Dyads: Synthesis and Photochemical Properties. Inorg. Chem. 2018, 57, 2977-2988.
(7) Meares, A.; Satraitis, A.; Ptaszek, M. BODIPY–Bacteriochlorin Energy Transfer Arrays: Toward Near-IR Emitters with Broadly Tunable, Multiple Absorption Bands. J. Org. Chem. 2017, 82, 13068-13075.
(8) Meares, A.; Satraitis, A.; Akhigbe, J.; Santhanam, N.; Swaminathan, S.; Ehudin, M.; Ptaszek, M. Amphiphilic BODIPY-Hydroporphyrin Energy Transfer Arrays with Broadly Tunable Absorption and Deep Red/Near-Infrared Emission in Aqueous Micelles. J. Org. Chem. 2017, 82, 6054-6070.
(9) Nopondo, E. N.; Yu, Z.; Wiratan, L.; Satraitis, A.; Ptaszek M.; Bacteriochlorin Dyads as Solvent Polarity Dependent Near-Infrared Fluorophores and Reactive Oxygen Species Photosensitizers. Org. Lett. 2016, 18, 4590-4593.
(10) Kang, H. S.; Esemoto, N. N.; Diers, J.; Niedzwiedzki, D.; Greco, J.; Akhigbe, J.; Yu, Z.; Pancholi, C.; Viswanathan B., G.; Nguyen, J. K.; Kirmaier, C.; Birge, R.; Ptaszek, M.; Holten, D.; Bocian, D. F. Effects of Strong Electronic Coupling in Chlorin and Bacteriochlorin Dyads J. Phys. Chem. A 2016, 120, 379-385.
(11) Meares, A.; Santhaman, N.; Satraitis, A. Yu, Z.; Ptaszek, M. Deep-red emissive BODIPY-chlorin arrays, excitable with green and deep-red light. J. Org. Chem. 2015, 80, 3858-3869.
(12) Ra, D.; Gauger, K. A.; Muthukumaran, K. ; Balasubramanian, B.; Chandrashaker, V.; Taniguchi, M.; Yu, Z.; Talley, D. C.; Ehudin, M.; Ptaszek, M.; Lindsey, J. S. Progress Towards Synthetic Chlorins with Graded Polarity, Conjugatable Substituents, and Wavelength Tunability. J. Porphyrins. Phthalocyanines. 2015, 19, 547-572.
(13) Yu, Z.; Pancholi, C.; Bhagavathy, G. V.; Kang, H, S.; Nguyen, J. K., Ptaszek, M. Strongly Conjugated Hydroporphyrin Dyads: Extensive Modification of Hydroporphyrins’ Properties by Expanding the Conjugated System J. Org. Chem. 2014, 79, 7910-7925.
(14) Harada, T.; Sano, K.; Sato, K.; Watanabe, R.; Yu, Z.; Hanaoka, H. Nakajima, T.; Choyke, P. L.; Ptaszek, M.; Kabayashi, H. Activatable Organic near-Infrared Fluorescent Probes Based on a Bacteriochlorin Platform: Synthesis and Multicolor in Vivo Imaging with a Single Excitation. Bioconjugate Chem. 2014, 25, 362-369.
(15) Yu, Z.; Ptaszek, M. “Near-IR Emissive Chlorin-Bacteriochlorin Energy-Transfer Dyads with a Common Donor and Acceptors with Tunable Emission Wavelength.” J. Org. Chem. 2013, 78, 10678-10691.
(16) Vinita, A. M.; Sano, K.; Yu, Z.; Nakajima, T.; Choyke, P.; Ptaszek, M.; Kobayashi, H. “Galactosyl human serum albumin-NMP1 conjugate: A near infrared-near (NIR)-activatable fluorescence imaging agent to detect peritoneal ovarian cancer metastases.” Bioconjugate Chem. 2012, 23, 1671-1679.
(17) Yu, Z.; Ptaszek, M. “Multifunctional Bacteriochlorins from selective palladium-coupling reactions.” Org. Lett. 2012, 14, 3708-3711.
Courses Taught
- CHEM 490: Fluorescence Sensing
- CHEM 684: Special Topics in Chemistry
- CHEM 351: Organic Chemistry I
- CHEM 352L: Organic Chemistry Lab II
- CHEM 690: Chemistry Seminar
- CHEM 713: Biochemistry/Chemistry Seminar